Bilirubin Screening & Testing in Newborns
On this Page
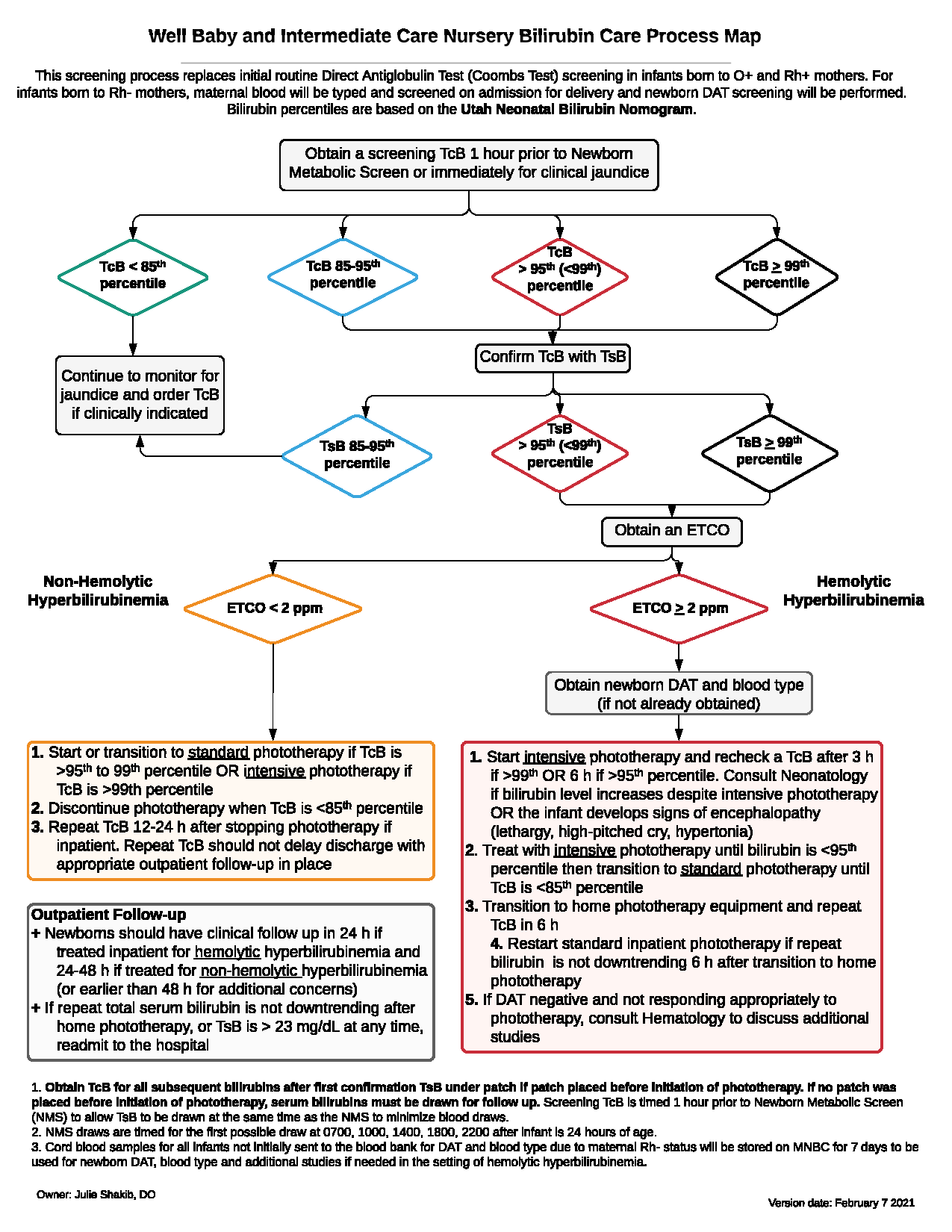
This 2021 care process map explains the current practice for screening and testing bilirubin in newborns in the University of Utah Hospital’s Well Baby and Intermediate Care Nurseries (WBN and ICN) based on the Utah Neonatal Bilirubin Nomogram found at [Bahr: 2021].
Key Points
- Infants ages 34 weeks and older in the WBN or ICN should be screened for hyperbilirubinemia around 24 hours of life with a transcutaneous bilirubin (TcB) or sooner if clinically jaundiced. A TcB may be used to track an infant’s bilirubin if a light occlusive patch is applied to the skin prior to initiating phototherapy.
- Risk stratification is determined using the Utah Neonatal Bilirubin Nomogram, an evidence-based alternative to the traditional Bhutani nomogram based on bilirubin levels for over 400,000 Utah births. Programmed into Epic, the Utah bilirubin Nomogram [Bahr: 2021] assist the postpartum care team to efficiently follow-up on infants with jaundice or hyperbilirubinemia.
- Further data, including total serum bilirubin (TsB), end-tidal carbon monoxide (ETCO), newborn blood type, and direct antibody testing (DAT), may be indicated.
- Based on the risk stratification, intensive, standard, or “home” phototherapy may be provided to infants with hyperbilirubinemia. Neonatology consultation may also be required for sick infants or those who do not respond to phototherapy as expected.
- Post-discharge outpatient follow-up guidance is provided up to 5 days of life, including for hemolytic and non-hemolytic infants with hyperbilirubinemia.
Authors & Reviewers
Initial publication: February 2023
Current Authors and Reviewers:
Page Bibliography
Bahr TM, Henry E, Christensen RD, Minton SD, Bhutani VK.
A New Hour-Specific Serum Bilirubin Nomogram for Neonates ≥35 Weeks of Gestation.
J Pediatr.
2021;236:28-33.e1.
PubMed abstract